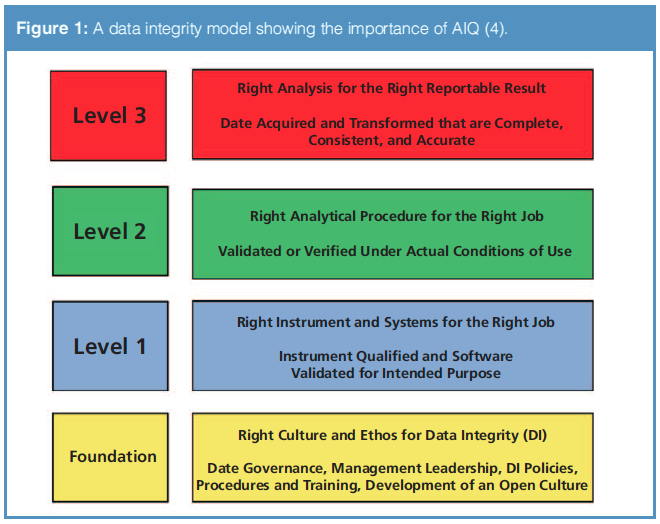
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records

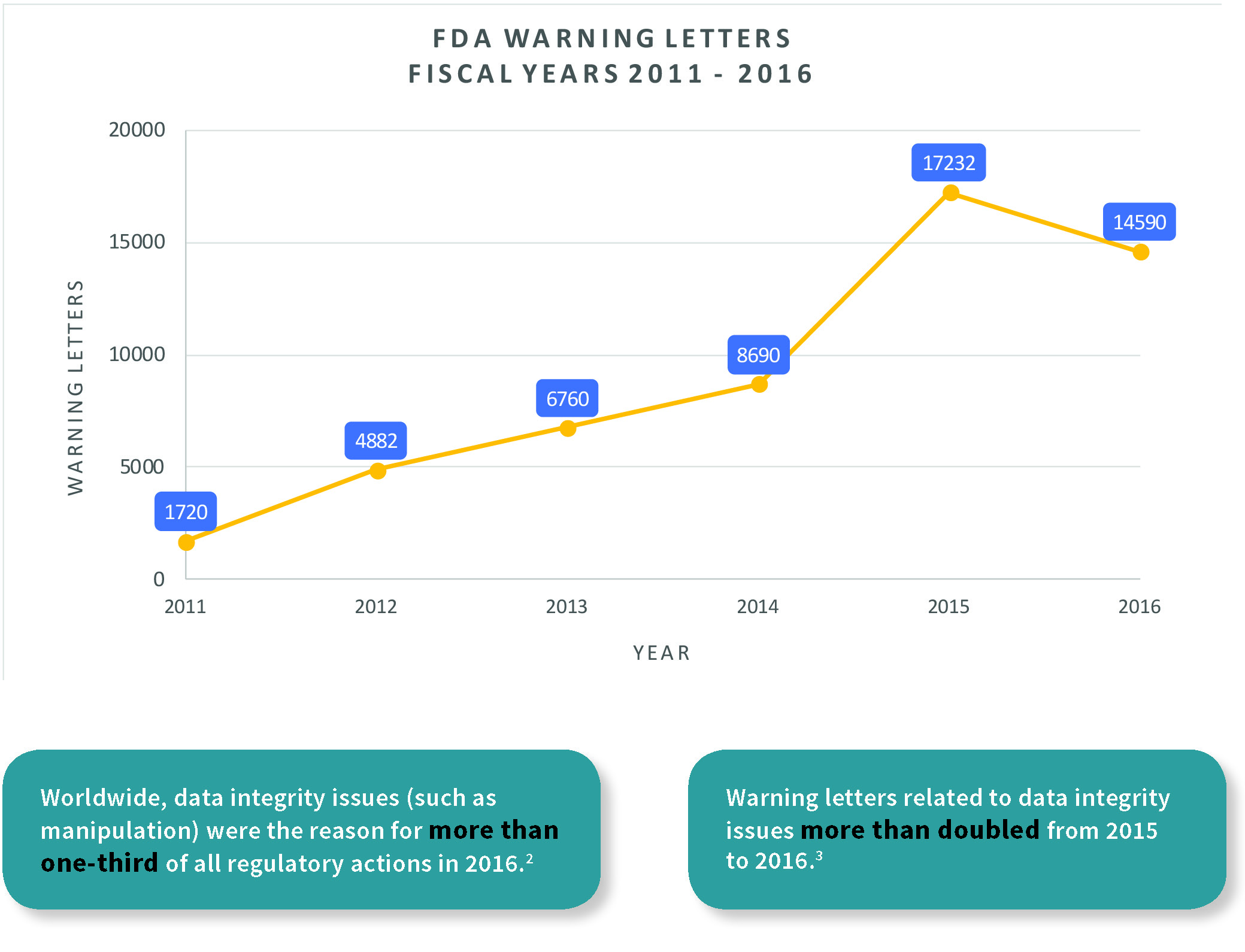
Pharmaceutical Data Integrity: issues, challenges and proposed solutions for manufacturers and inspectors - GaBI Journal

Data Integrity Awareness: Good Documentation Practices - LearnGxP: Accredited Online Life Science Training Courses
Are You Ready for the Latest Data Integrity Guidance? Part 1: Scope, Data Governance, and Paper Records

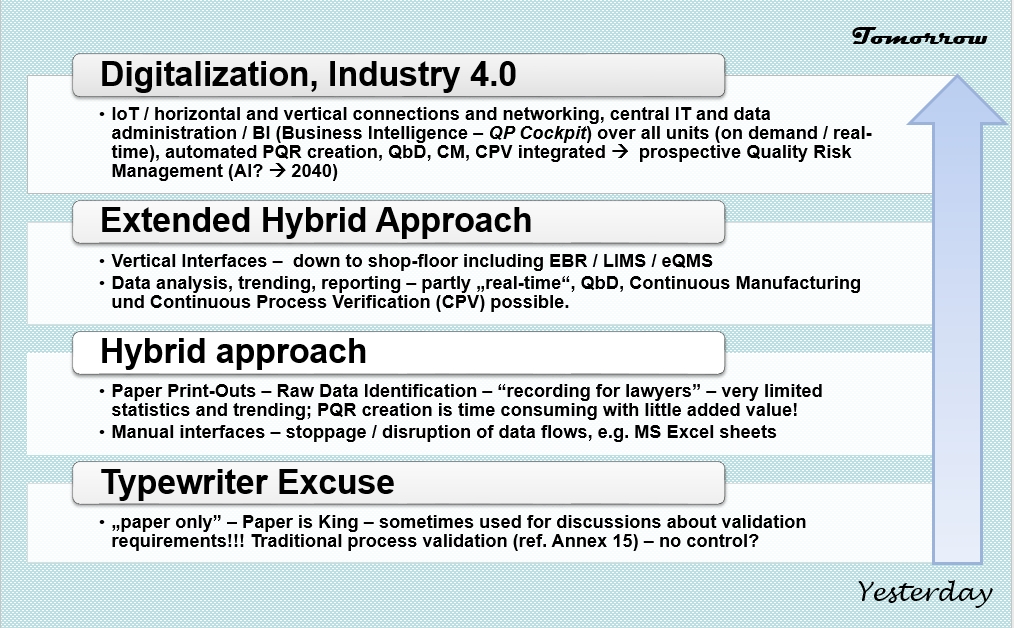
Data integrity within the biopharmaceutical sector in the era of Industry 4.0 - Alosert - 2022 - Biotechnology Journal - Wiley Online Library
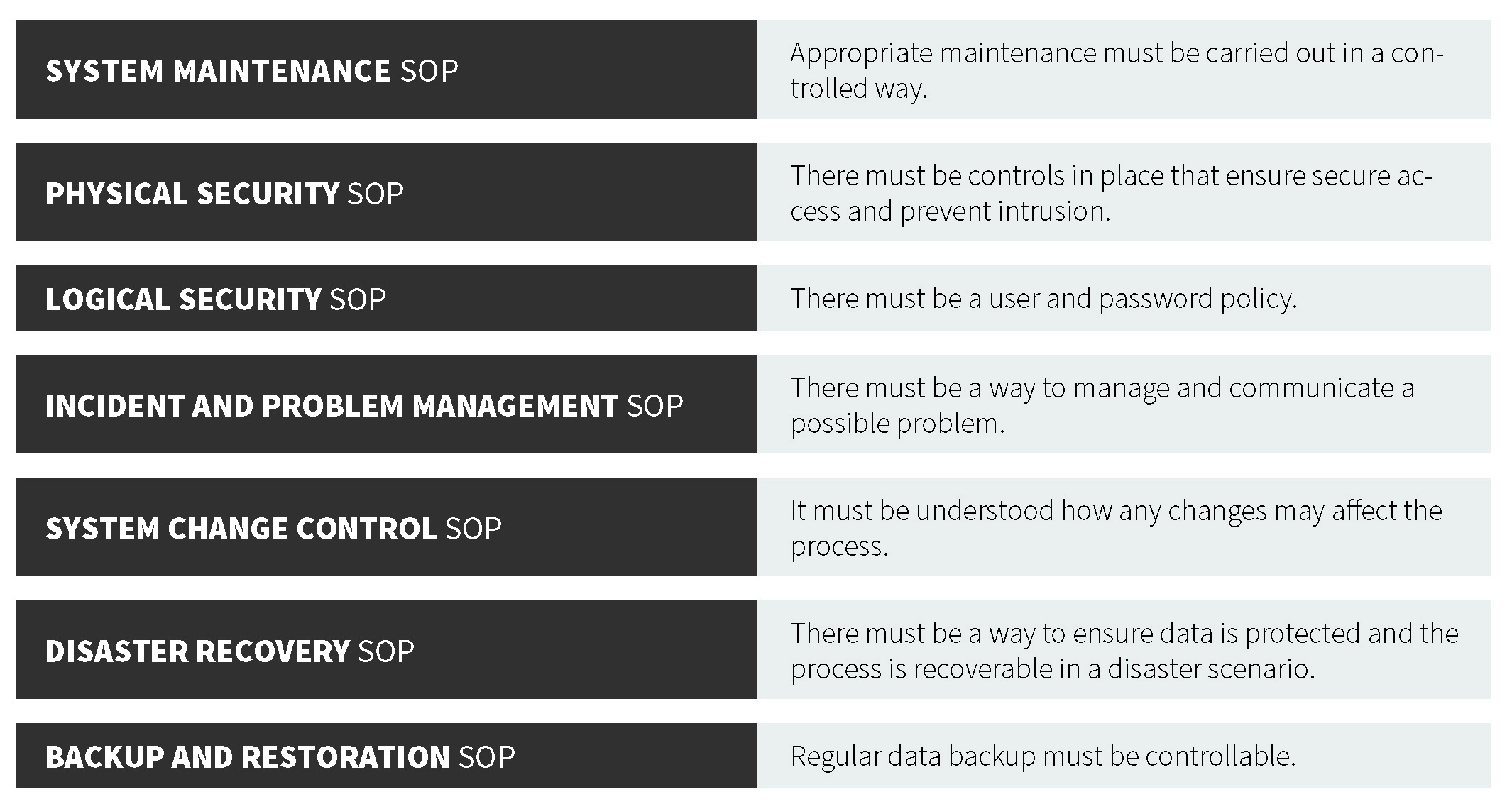
![8 Proven Strategies to Ensure IT Backup Data Integrity [Video] - LearnGxP: Accredited Online Life Science Training Courses 8 Proven Strategies to Ensure IT Backup Data Integrity [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://embed-ssl.wistia.com/deliveries/718dc73d8b867e515db6a1f78198cc682bf77aa3.jpg?image_crop_resized=640x360)